Spodumeneis a single-stranded silicate belonging to the pyroxene group. The stoichiometric composition of pure spodumene is Li2O (8%), Al2O3(27.4%) and SiO2(64.6%) . The crystal structure of spodumene appears as α-spodumene under low-temperature (stable), β-spodumene (stable) and γ-spodumene (metastable) under high-temperature during calcination. The three crystal structures of spodumene are shown as below.



α-spodumene β-spodumene γ-spodumene
To systematically analyze the thermal stability and composition of spodumene, we use TG-DSC instruments to test the quality changes with different temperature, and the test results are shown as below.
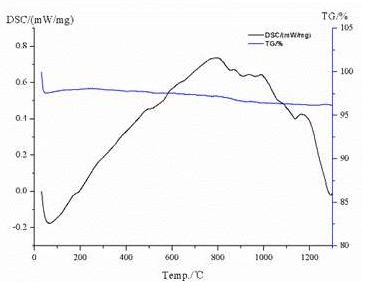
The materials are accompanied by an endothermic processwhen they are volatilized and decomposed, while accompanied by an exothermic process incombination reactions. The endothermic peak at about 150 °C, and the inner water of the spodumene crystal began to remove. There is an endothermic peak on the DSC curve, and a loss of weight on the TG curve. The energy change is fierce while the weight change is weak, which is a sign of the combination reaction and crystal transition of the materials. Therefore, several exothermic peaks on the DSC curve in the temperature range of 800-1200 ℃ can be judged as the form of β-spodumene.
According to TG-DSC test results, our institute designed a sintering system of spodumene calcination process. That is: heating up start from room temperature, at the rate of10 ℃/min, and then keep for 30mins at the temperature of 1150 ℃. And then, take out the crucible with quenched, grind the materials to fine and test the mineral compositions when cooled to room temperature. The results of two different spodumene experiments are as follows,


The XRD results showed that the main crystal transition of spodumene was good. The ore was mainly non-transformed α-spodumene and a certain amount of silica. And they all transformed into β-spodumene at 1150 ℃ during the calcination.
Next, our institute will study in-depth of the spodumene leaching experiment, select the appropriate acidification time and sulfuric acid ratio, in order to achieve higher lithium leaching rate.